- HOME
- English
English
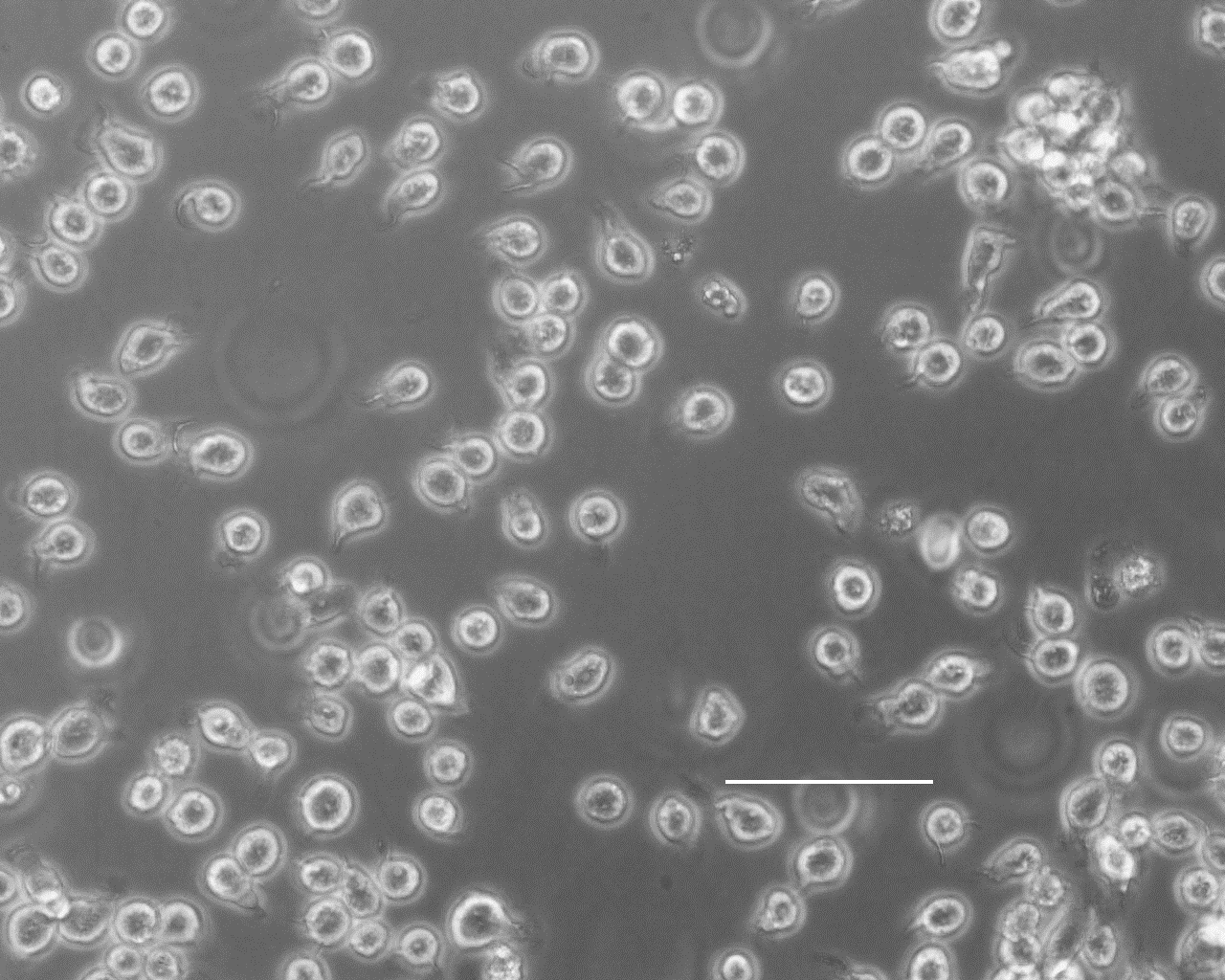
THP-G8 cells are utilized for the in vitro skin sensitisation test (IL-8 Luc Assay), which is approved as an internationally recognized testing protocol by the Organization for Economic Co-operation and Development (OECD).
The THP-G8 cells are derived from human acute monocytic leukemia cell line (THP-1 cells) stably introducing luciferase genes, in which orange-emitting luciferase (Stable Luciferase Orange, SLO) is expressed under the control of interleukin-8 (IL-8) promoter, and red-emitting luciferase (Stable Luciferase Red, SLR) is expressed under the control of GAPDH promoter, used as an internal control reporter.
To evaluate skin sensitisation, transcriptional activation of interleukin-8 (IL-8) promoter by test chemicals, seen in human dendritic cells in skin, is simply measured by luciferase reporter assay.
* THP-G8 cells were established by Department of Dermatology, Tohoku University School of Medicine (Takahashi T. et al. Toxicol. Sci., 124, 359-369, 2011).
1.Availability and Cost
The IL-8 Luciferase Reporter Cell Line is provided on a fee-for-service basis through an authorized distributor.
Pricing (via distributor; tax excluded, shipping not included)
- For-profit organizations
Initial license and material transfer fee (first two years, including one vial): USD 3,000
Maintenance fee (from the third year onward, for two years, without material transfer):USD 2,400 - Non-profit organizations
Initial license and material transfer fee (first two years, including one vial): USD 1,300
Maintenance fee (from the third year onward, for two years, without material transfer): USD 700 - Additional supply of cell line (all organization types)
USD 900 per vial
2.Conditions of Use
The cell line may be used for testing and evaluation in accordance with OECD TG 442E.
Clinical, diagnostic, or therapeutic use is prohibited.
Use of the cell line is subject to the applicable contractual terms.
3.How to Obtain the Cell Line
The cell line is available through the following authorized distributor:
Distributor
POC Inc.
4.Requirement for MTA
Execution of a Material Transfer Agreement (MTA) is mandatory prior to distribution.
5. License Agreement (Additional Note)
For commercial-related use, a separate license agreement may be required, as applicable.
References
- Takahashi T. et al., (2011) An in vitro test to screen skin sensitizers using a stable THP-1-derived IL-8 reporter cell line, THP-G8. Toxicol. Sci., 124, 359-369.
- Kimura Y. et al., (2015) Optimization of the IL-8 Luc assay as an in vitro test for skin sensitization. Toxicol. In Vitro, 29, 1816-1830.
- Kimura Y. et al., (2018) Profiling the immunotoxicity of chemicals based on in vitro evaluation by a combination of the Multi-ImmunoTox assay and the IL-8 Luc assay. Arch. Toxicol., 92, 2043-2054.
- Kimura Y. et al., (2018) The performance of an in vitro skin sensitisation test, IL-8 Luc assay (OECD442E), and the integrated approach with direct peptide reactive assay (DPRA). J. Toxicol. Sci., 43, 741-749.
- Kimura Y. et al., (2021) The modified IL-8 Luc assay, an in vitro skin sensitisation test, can improve the false-negative judgment of lipophilic sensitizers with logKow values > 3.5. Arch. Toxicol., 95, 749-758.
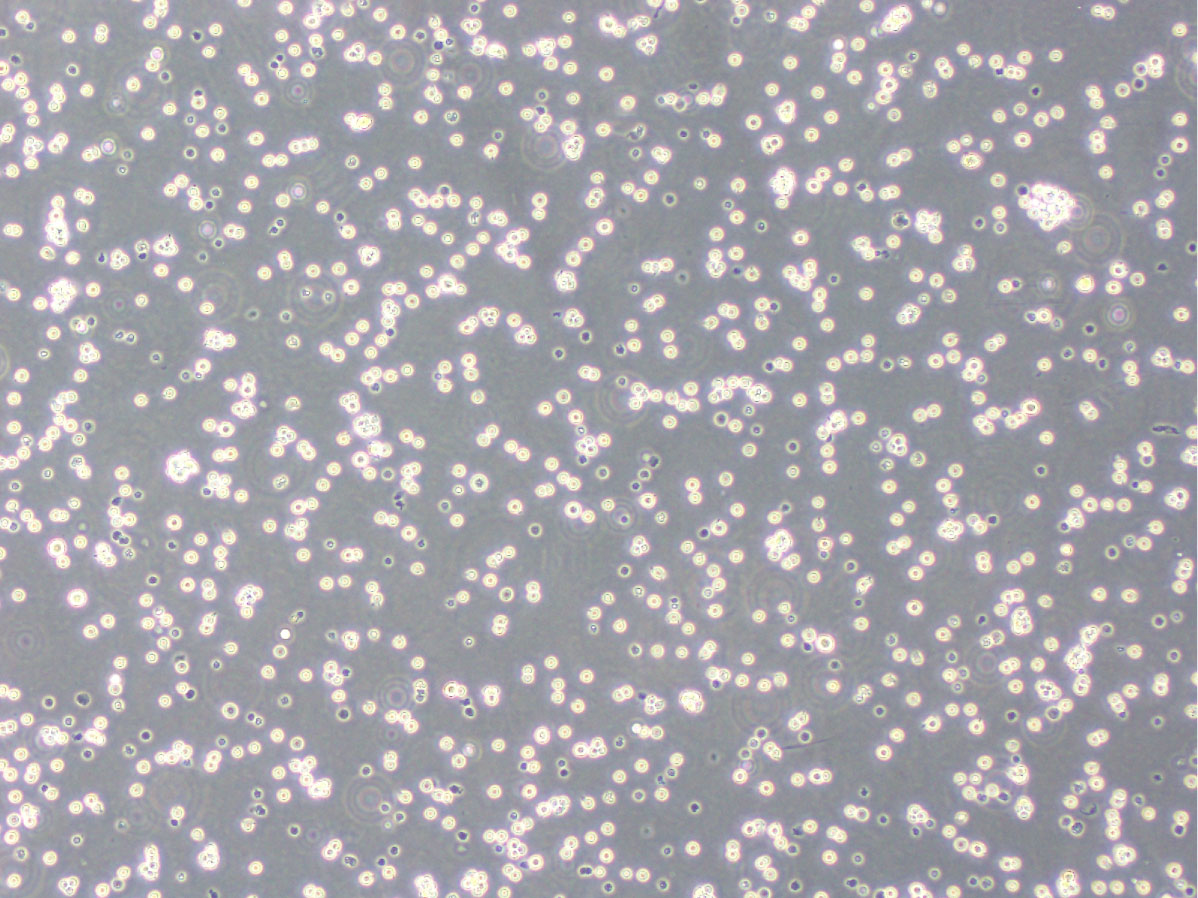
2H4 cells are utilized for the in vitro immunotoxicity test (IL-2 Luc Assay), which is approved as an internationally recognized testing protocol by the Organization for Economic Co-operation and Development (OECD).
2H4 cells are derived from human acute T lymphoblastic cell line (Jurkat cells) stably introducing luciferase genes, in which green-emitting luciferase (Stable Luciferase Green, SLG) is expressed under the control of interleukin-2 (IL-2) promoter, orange-emitting luciferase (Stable Luciferase Orange, SLO) is expressed under the control of interferon (IFN)- γ promoter, and red-emitting luciferase (Stable Luciferase Red, SLR) is expressed under the control of GAPDH promoter, used as an internal control reporter.
To evaluate immunotoxicity, transcriptional change of IL-2 promoter of human T cells by immunotoxic chemicals is simply measured by luciferase reporter assay.
*2H4 cells were established by Tsuruga Institute of Biotechnology, TOYOBO CO., LTD., and Department of Dermatology, Tohoku University School of Medicine (Saito R. et al.(2011) Toxicol. Appl. Pharmacol., 254, 245-255).
1. Availability and Cost
The 2H4 cell line is provided on a fee-for-service basis through an authorized distributor.
Pricing (via distributor; tax excluded, shipping not included)
- For-profit organizations
Initial license and material transfer fee (first two years, including one vial):USD 3,000
Maintenance fee (from the third year onward, for two years, without material transfer): USD 2,400 - Non-profit organizations
Initial license and material transfer fee (first two years, including one vial): USD 1,300
Maintenance fee (from the third year onward, for two years, without material transfer):USD 700 - Additional supply of cell line (all organization types)
USD 900 per vial
2. Conditions of Use
The cell line may be used for testing and evaluation in accordance with OECD TG 444A (IL-2 Luc Assay / IL-2 Luc LTT).
Clinical, diagnostic, or therapeutic use is prohibited.
Use of the cell line is subject to the applicable contractual terms.
3. How to Obtain the Cell Line
The cell line is available through the following authorized distributor:
Distributor
POC Inc.
4. Requirement for MTA
Execution of a Material Transfer Agreement (MTA) is mandatory prior to distribution.
5. License Agreement (Additional Note)
For commercial-related use, a separate license agreement may be required, as applicable.
References
- Saito, R. et al. (2011) Nickel differentially regulates NFAT and NF-kappaB activation in T cell signaling. Toxicol. Appl. Pharmacol., 254, 245-255.
- Kimura Y. et al., (2020) An international validation study of the IL-2 Luc assay for evaluating the potential immunotoxic effects of chemicals on T cells and a proposal for reference data for immunotoxic chemicals. Toxicol. In Vitro, 66, 104832.
- Kimura Y. et al., (2021) Optimization of the IL-2 Luc assay for immunosuppressive drugs: a novel in vitro immunotoxicity test with high sensitivity and predictivity. Arch. Toxicol., 95, 2755-2768.
- Kimura Y. et al., (2023) An international validation study of the interleukin-2 luciferase leukocyte toxicity test (IL-2 Luc LTT) to evaluate potential immunosuppressive chemicals and its performance after use with the interleukin-2 luciferase assay (IL-2 Luc assay). Toxicol. In Vitro, 88, 105535.


